
The quantum-mechanical explanation of valence is more detailed and correspondingly more powerful than the old picture. In 1928, Pauling tried to relate this newly created quantum mechanics with valence.
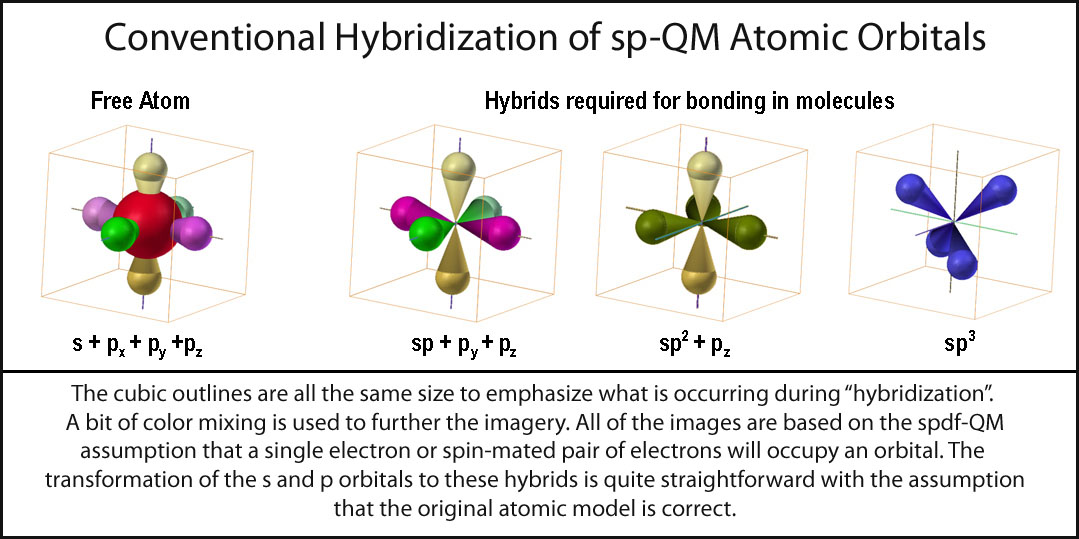
In a brilliant intellectual achievement, Schroedinger developed wave mechanics in four papers published in 1926 with the immediately preceding matrix mechanics of Heisenberg and the symbolic method of Pauli, these articles were the foundations of quantum mechanics. Lewis associated a chemical bond with a pair of electrons between adjacent atomic nuclei, but Bohr recognized that a static distribution of charges must be unstable if Coulomb’s law that a force is inversely proportional to a distance between two electric charges is applicable. Thomson’s discovery of the electron in 1897, his association of an electron with the structure of an atom originated a connection between an electron and bonding, later reinforced by Rutherford’s construction of the nuclear atom. Within the next two decades, to explain optical activity van’t Hoff and Lebel in 1873 concurrently deduced the idea of directed valences in the form of a carbon atom having tetrahedrally oriented neighboring atoms. In 1852 Edward Frankland had already published a paper that proposed the idea of chemical valencies, hence discovering the chemical bond. Couper’s idea was that carbon atoms can link to each other following valence regularities. Half a century after Dalton’s atomic hypothesis, Archibald Scott Couper, a Scottish chemist working in Paris, proposed the first enduring notions about molecular structure: " New Chemical Theory" in 1858. Hybrid atomic orbitals (HAO) are perceived to arise from a culmination of two convergent themes in scientific research, one chemical and the other physical, beginning in the nineteenth century. This review is not impartial as all previous reviews were biased toward support of hybridization, we contend that it is time to review all previous evidence of hybridization through a critical eye. Nearly a century has elapsed since the discovery of the process of hybridization of atomic orbitals a critical review is essential to organize the pertinent information in the twenty-first century. This review of hybrid atomic orbitals and hybridization is inspired by our recent work on a critique of these hybrid orbitals. Even though hybrid orbitals might have fulfilled a role as crude models in the twentieth century, we predict that they will not survive in this new century as more complicated models are required and applied.

Based on a critical survey of the literature, including the latest experimental results and our results, we summarize the main points of using hybridization from the past and why hybridization should be eliminated from organic chemistry.
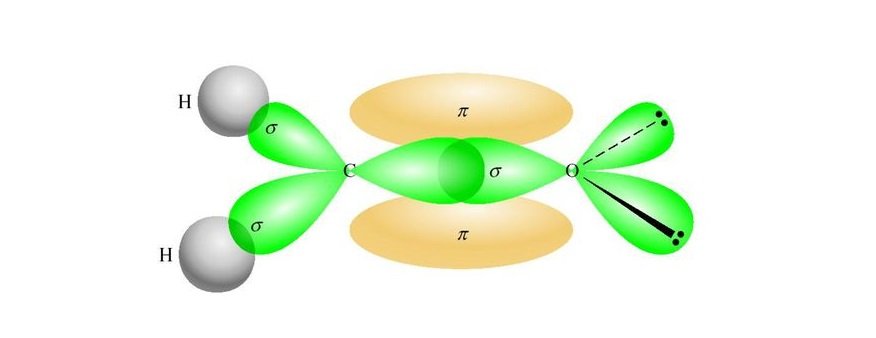
The confusion among various descriptions of ‘hybridization’, which has perplexed generations of chemists, is clarified. The history of these hybrids and hybridization as a process is discussed this history is divided into a timeline from before 1931 to the present day. Hybrid atomic orbitals have become a central component of organic chemistry.


 0 kommentar(er)
0 kommentar(er)
